Service offer
Viralgen specializes in the production of AAV gene therapy vectors using our proprietary suspension, triple transfection platform. This includes a HEK-293-derived suspension cell line, a scalable upstream and robust purification process, coupled with full support for fill and finish, QC testing, and regulatory support from preclinical to commercial requirements.
Come to us with your GOI and POTENCY ASSAY and we'll carry out the development, production and management of your product in a more secure and efficient way.

Scale and suites
We offer multiple scales and grades of manufacture to help you meet the needs of a gene therapy program throughout product's life-cycle.
Feasibility at 2L scale:
The 2L feasibility study we offer at Viralgen is an important step in enabling our customers to make data-driven decisions about working with us and the needs of their programs, without requiring a long-term commitment.
The study will deliver a small amount (1-2mL) of material as well as productivity, yield and purity data. This enables you as a client to get a quick evaluation of your construct in our platform and projections on future scale-up. This process also allows us to evaluate if your construct will require any potential process optimization prior to moving to the next scale.
We have put together a short video below explaining why these studies are so important, and the value they bring to your decisions as a client.

Research or toxicology at 50L scale
Based of the data from your 2L study we can quickly move into the 50L scale. Here, we offer 50L bioreactors to manufacture to supply material for larger research evaluations, toxicology studies, biodistribution studies, stability programs, and other late preclinical needs.
Clinical cGMP
We can supply you between 250L and 2000L of clinical-grade AAV from bioreactors ranging between 250 and 2000L.
Experience
Since opening in 2018, Viralgen has been busy making AAV for clients all over the world. At the end of 2022, we have produced more than 650 batches to customers and more than 50 batches, and have supported multiple clinical trials in the US, Canada and Europe. Our focus on AAV and our platform deepens our understanding of AAV every day. Let us help you leverage from our experience.
VIRALGEN'S EXPERIENCE | We produced > 950 batches, with a range of different Serotypes
Viralgen | 2018 - Today
| Batches produced |
|
|---|---|
| Research Batches (2L) | 937 |
| Toxicology Batches (50L) | 79 |
| Clinical cGMP Batches (250L | 500L) | 91 |
| Commercial cGMP Batches (2000L) | 11 |
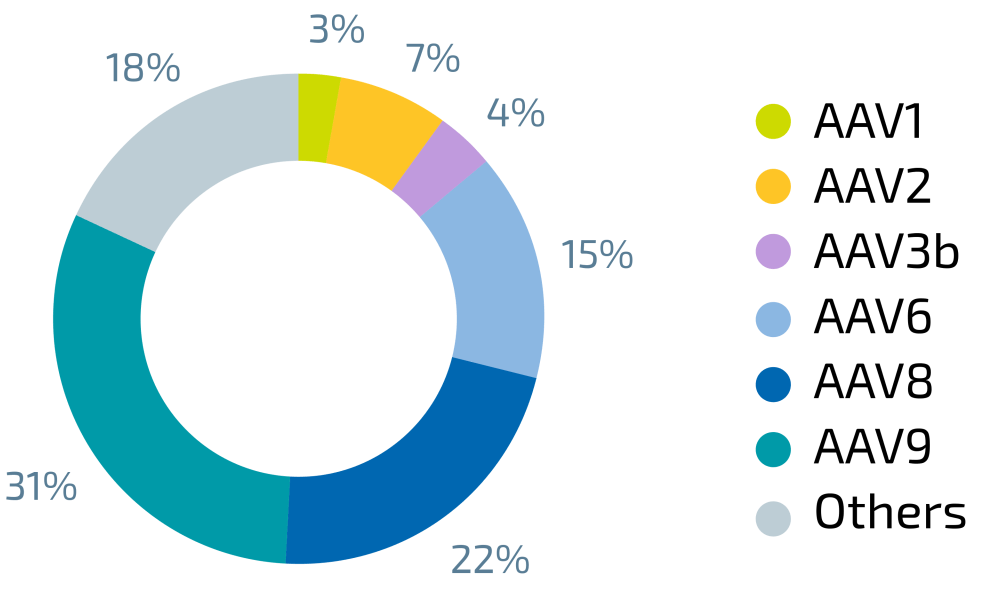



Let’s talk
Do you want to know more about how we can help you?
 Parque Científico y
Tecnológico de Gipuzkoa,
Paseo Mikeletegi 83,
20009 San Sebastián,
Spain
Parque Científico y
Tecnológico de Gipuzkoa,
Paseo Mikeletegi 83,
20009 San Sebastián,
Spain Tel:
Tel:
 Press contact:
Press contact: