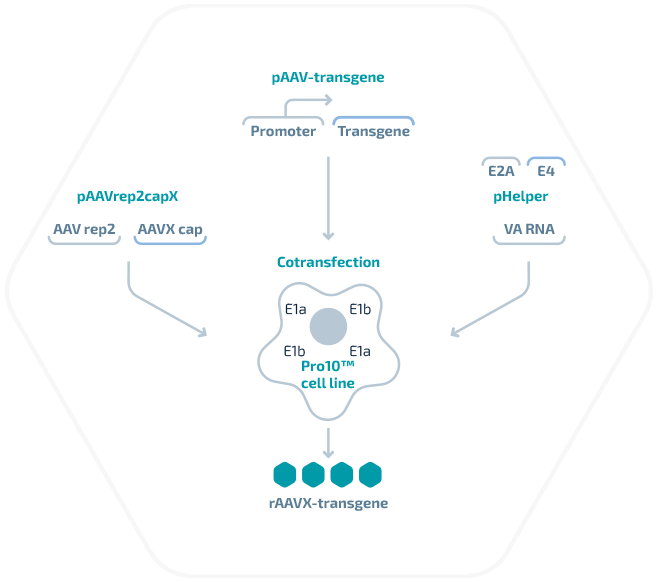
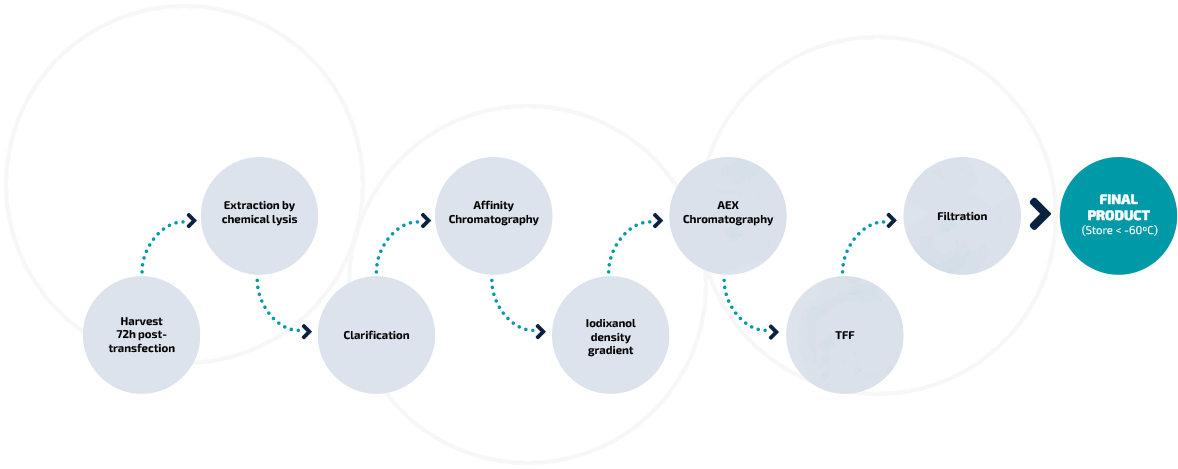
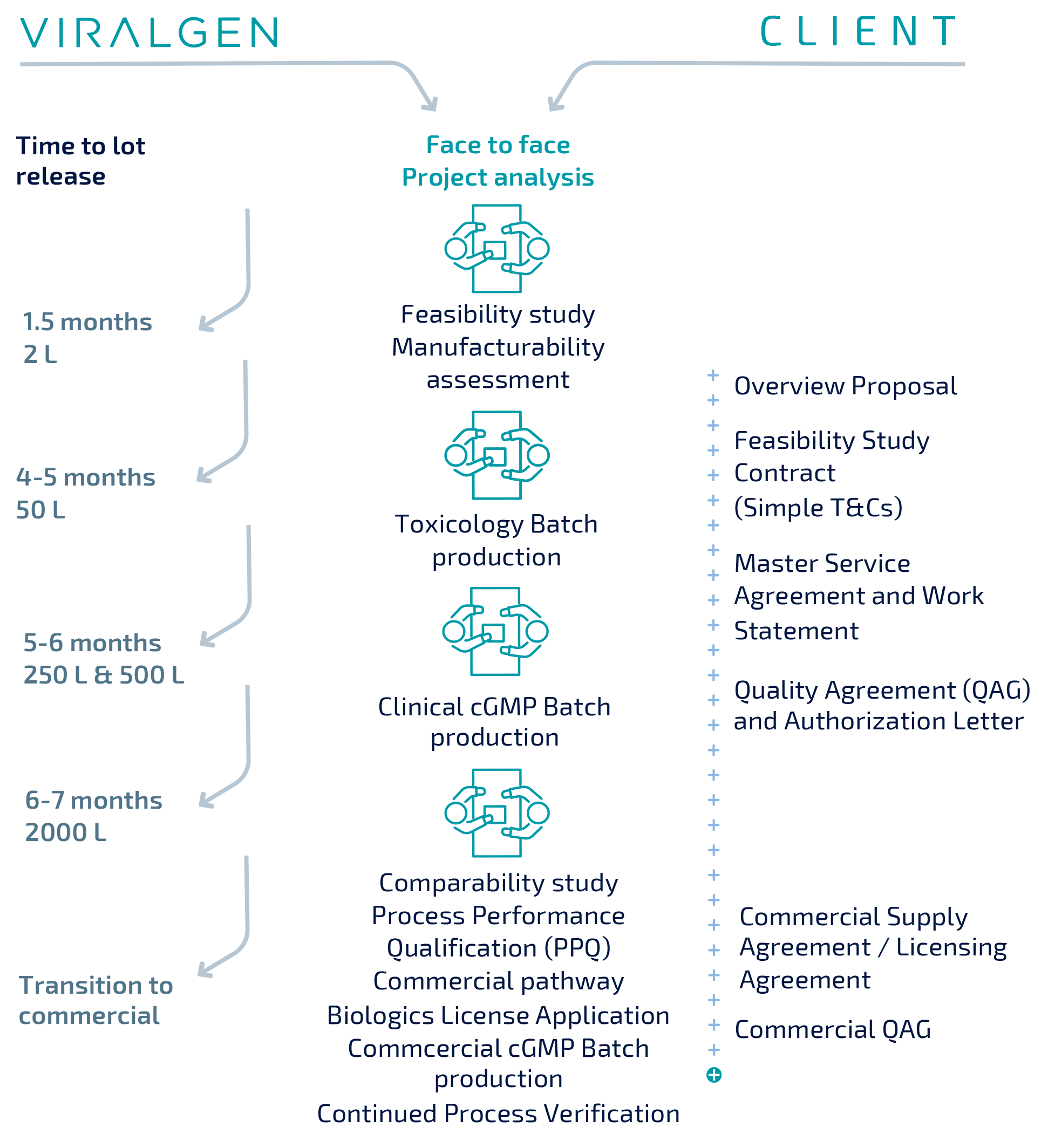
Regulatory support
Regulatory interactions in gene therapy are complex and can be challenging – as our understanding of AAV grows so too does the challenges of submission and approval of these therapeutics.
At Viralgen, we are committed to your success in those interactions with regulatory agencies. As a part of our inclusive pricing, we are happy to provide you with whatever level of support required for the Chemistry, Manufacturing and Control (CMC) areas of each submission – from providing template articles to authoring them, and participation in information exchanges with regulators either in writing or in person.
 Parque Científico y
Tecnológico de Gipuzkoa,
Paseo Mikeletegi 83,
20009 San Sebastián,
Spain
Parque Científico y
Tecnológico de Gipuzkoa,
Paseo Mikeletegi 83,
20009 San Sebastián,
Spain Tel:
Tel:
 Press contact:
Press contact: