Your new horizon
In aav gene therapy (cGMP) manufacturing at scale
Looking to manufacture gene therapy on a large scale?
Currently producing at 2000L in our commercial cGMP production facility
Find out why we are your best partner
Reasons why you will be at the forefront
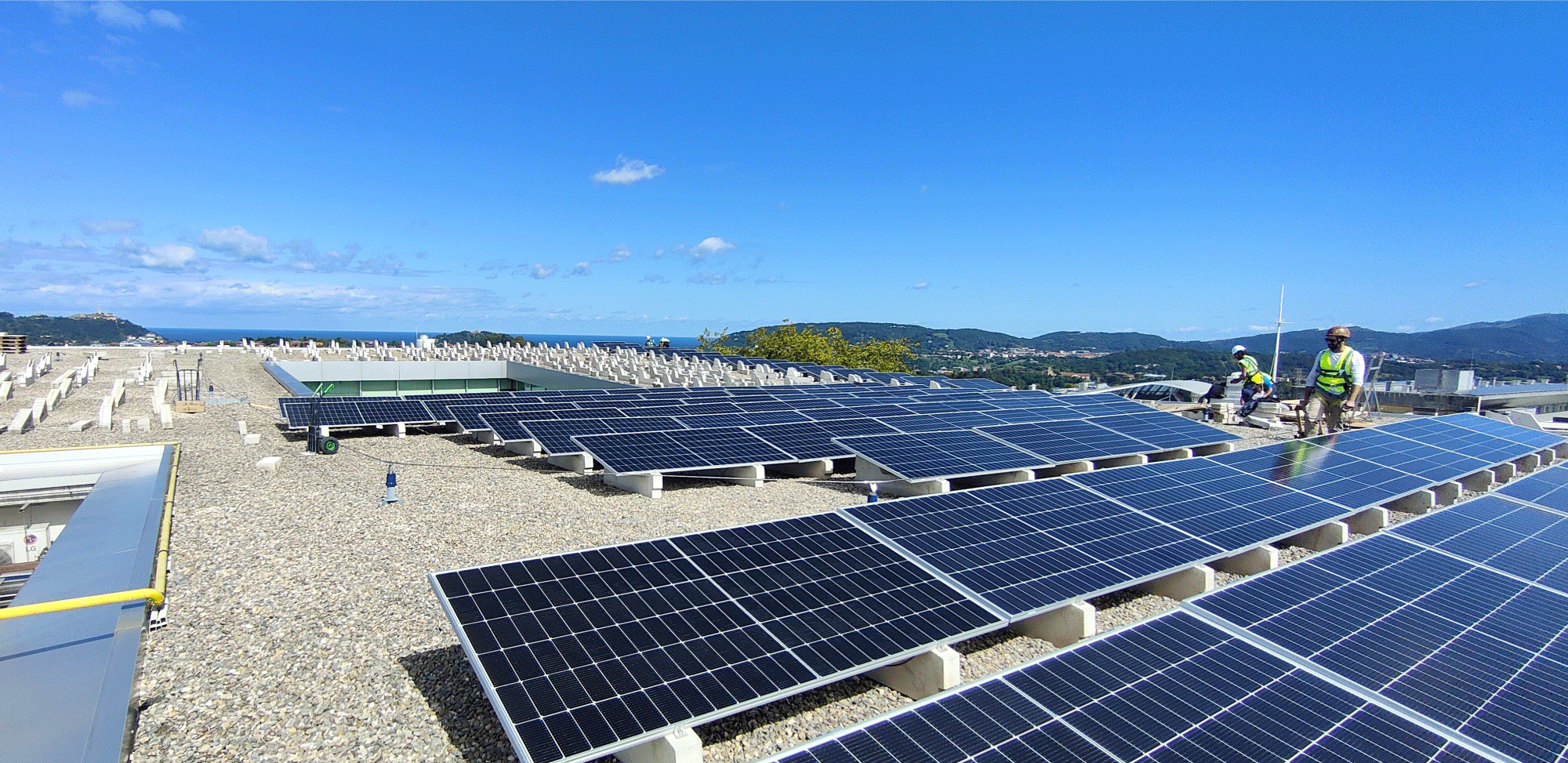
You can count on cutting-edge large-scale cGMP capacity
You can manufacture 7 different products at the same time in 7 independent state-of-the-art cGMP manufacturing rooms
- 50L scale to supply toxicology/biodistribution studies.
- 4 independent state-of-the-art cGMP clinical production suites, providing the capability to continuously manufacture 4 different products simultaneously.
- Viralgen uses 250L and 500L single-use stirred-tank bioreactors for culture of suspension cells in clinical manufacturing.
- Currently producing at 2000L in our commercial cGMP production facility.
- In-house QC lab for critical release assays.
You will benefit from our extensive expertise in AAV and its regulations throughout the whole process of development and production
A world-class highly-motivated professional team will be at your disposal. They will guide you throughout the whole process, from development to large-scale up to 2000L production and commercialization.
You will have greater internal control over time and cost.
Viralgen allows for the rapid incorporation of new programs, which will accelerate your process.
Optimization will increase by more than 70%
Viralgen has a proprietary mammalian cell-based production platform, which results in an optimization rate of more than 70% compared to other cell lines.
Viralgen has the license for AskBio’s Pro10™ cell line, which is derived from HEK293 cells and produces high yields. It has also licensed a new one-of-a-kind large-scale rAAV manufacturing process up to 2000L.
It is an industrial-scale serum-free suspension culture system. The manufacturing process is based on the triple transfection of the Pro10™ cell line.
SV40 sequences are not present in Pro10™ cell line.
Social Responsibility
What moves us
News
This is where you can keep up to date on the different activities and news of our organization.
Let’s talk
Do you want to know more about how we can help you?
 Parque Científico y
Tecnológico de Gipuzkoa,
Paseo Mikeletegi 83,
20009 San Sebastián,
Spain
Parque Científico y
Tecnológico de Gipuzkoa,
Paseo Mikeletegi 83,
20009 San Sebastián,
Spain Tel:
Tel:
 Press contact:
Press contact: